Biology
| Part of a series on |
| Biology |
|---|
Biology is the scientific study of life.[1][2][3] It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field.[1][2][3] For instance, all organisms are made up of cells that process hereditary information encoded in genes, which can be transmitted to future generations. Another major theme is evolution, which explains the unity and diversity of life.[1][2][3] Energy processing is also important to life as it allows organisms to move, grow, and reproduce.[1][2][3] Finally, all organisms are able to regulate their own internal environments.[1][2][3][4][5]
Biologists are able to study life at multiple levels of organization,[1] from the molecular biology of a cell to the anatomy and physiology of plants and animals, and evolution of populations.[1][6] Hence, there are multiple subdisciplines within biology, each defined by the nature of their research questions and the tools that they use.[7][8][9] Like other scientists, biologists use the scientific method to make observations, pose questions, generate hypotheses, perform experiments, and form conclusions about the world around them.[1]
Life on Earth, which emerged more than 3.7 billion years ago,[10] is immensely diverse. Biologists have sought to study and classify the various forms of life, from prokaryotic organisms such as archaea and bacteria to eukaryotic organisms such as protists, fungi, plants, and animals. These various organisms contribute to the biodiversity of an ecosystem, where they play specialized roles in the cycling of nutrients and energy through their biophysical environment.
History
The earliest of roots of science, which included medicine, can be traced to ancient Egypt and Mesopotamia in around 3000 to 1200 BCE.[11][12] Their contributions later entered and shaped Greek natural philosophy of classical antiquity.[11][12][13][14] Ancient Greek philosophers such as Aristotle (384–322 BCE) contributed extensively to the development of biological knowledge. His works such as History of Animals were especially important because they revealed his naturalist leanings, and later more empirical works that focused on biological causation and the diversity of life. Aristotle's successor at the Lyceum, Theophrastus, wrote a series of books on botany that survived as the most important contribution of antiquity to the plant sciences, even into the Middle Ages.[15]
Scholars of the medieval Islamic world who wrote on biology included al-Jahiz (781–869), Al-Dīnawarī (828–896), who wrote on botany,[16] and Rhazes (865–925) who wrote on anatomy and physiology. Medicine was especially well studied by Islamic scholars working in Greek philosopher traditions, while natural history drew heavily on Aristotelian thought, especially in upholding a fixed hierarchy of life.
Biology began to quickly develop and grow with Anton van Leeuwenhoek's dramatic improvement of the microscope. It was then that scholars discovered spermatozoa, bacteria, infusoria and the diversity of microscopic life. Investigations by Jan Swammerdam led to new interest in entomology and helped to develop the basic techniques of microscopic dissection and staining.[17]
Advances in microscopy also had a profound impact on biological thinking. In the early 19th century, a number of biologists pointed to the central importance of the cell. Then, in 1838, Schleiden and Schwann began promoting the now universal ideas that (1) the basic unit of organisms is the cell and (2) that individual cells have all the characteristics of life, although they opposed the idea that (3) all cells come from the division of other cells. However, Robert Remak and Rudolf Virchow were able to reify the third tenet, and by the 1860s most biologists accepted all three tenets which consolidated into cell theory.[18][19]
Meanwhile, taxonomy and classification became the focus of natural historians. Carl Linnaeus published a basic taxonomy for the natural world in 1735 (variations of which have been in use ever since), and in the 1750s introduced scientific names for all his species.[20] Georges-Louis Leclerc, Comte de Buffon, treated species as artificial categories and living forms as malleable—even suggesting the possibility of common descent. Although he was opposed to evolution, Buffon is a key figure in the history of evolutionary thought; his work influenced the evolutionary theories of both Lamarck and Darwin.[21]
Serious evolutionary thinking originated with the works of Jean-Baptiste Lamarck, who was the first to present a coherent theory of evolution.[23] He posited that evolution was the result of environmental stress on properties of animals, meaning that the more frequently and rigorously an organ was used, the more complex and efficient it would become, thus adapting the animal to its environment. Lamarck believed that these acquired traits could then be passed on to the animal's offspring, who would further develop and perfect them.[24] However, it was the British naturalist Charles Darwin, combining the biogeographical approach of Humboldt, the uniformitarian geology of Lyell, Malthus's writings on population growth, and his own morphological expertise and extensive natural observations, who forged a more successful evolutionary theory based on natural selection; similar reasoning and evidence led Alfred Russel Wallace to independently reach the same conclusions.[25][26] Darwin's theory of evolution by natural selection quickly spread through the scientific community and soon became a central axiom of the rapidly developing science of biology.
The basis for modern genetics began with the work of Gregor Mendel, who presented his paper, "Versuche über Pflanzenhybriden" ("Experiments on Plant Hybridization"), in 1865,[27] which outlined the principles of biological inheritance, serving as the basis for modern genetics.[28] However, the significance of his work was not realized until the early 20th century when evolution became a unified theory as the modern synthesis reconciled Darwinian evolution with classical genetics.[29] In the 1940s and early 1950s, a series of experiments by Alfred Hershey and Martha Chase pointed to DNA as the component of chromosomes that held the trait-carrying units that had become known as genes. A focus on new kinds of model organisms such as viruses and bacteria, along with the discovery of the double-helical structure of DNA by James Watson and Francis Crick in 1953, marked the transition to the era of molecular genetics. From the 1950s onwards, biology has been vastly extended in the molecular domain. The genetic code was cracked by Har Gobind Khorana, Robert W. Holley and Marshall Warren Nirenberg after DNA was understood to contain codons. Finally, the Human Genome Project was launched in 1990 with the goal of mapping the general human genome. This project was essentially completed in 2003,[30] with further analysis still being published. The Human Genome Project was the first step in a globalized effort to incorporate accumulated knowledge of biology into a functional, molecular definition of the human body and the bodies of other organisms.
Chemical basis
Atoms and molecules
All organisms are made up of chemical elements;[31] oxygen, carbon, hydrogen, and nitrogen account for 96%[further explanation needed] of all organisms, with calcium, phosphorus, sulfur, sodium, chlorine, and magnesium constituting essentially all the remainder. Different elements can combine to form compounds such as water, which is fundamental to life.[31] Biochemistry is the study of chemical processes within and relating to living organisms. Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including molecular synthesis, modification, mechanisms, and interactions.
Water
Life arose from the Earth's first ocean, which was formed approximately 3.8 billion years ago.[32] Since then, water continues to be the most abundant molecule in every organism. Water is important to life because it is an effective solvent, capable of dissolving solutes such as sodium and chloride ions or other small molecules to form an aqueous solution. Once dissolved in water, these solutes are more likely to come in contact with one another and therefore take part in chemical reactions that sustain life.[32]
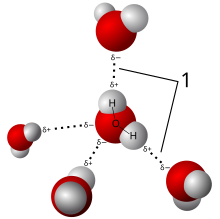
In terms of its molecular structure, water is a small polar molecule with a bent shape formed by the polar covalent bonds of two hydrogen (H) atoms to one oxygen (O) atom (H2O).[32] Because the O–H bonds are polar, the oxygen atom has a slight negative charge and the two hydrogen atoms have a slight positive charge.[32] This polar property of water allows it to attract other water molecules via hydrogen bonds, which makes water cohesive.[32] Surface tension results from the cohesive force due to the attraction between molecules at the surface of the liquid.[32] Water is also adhesive as it is able to adhere to the surface of any polar or charged non-water molecules.[32]
Water is denser as a liquid than it is as a solid (or ice).[32] This unique property of water allows ice to float above liquid water such as ponds, lakes, and oceans, thereby insulating the liquid below from the cold air above.[32] The lower density of ice compared to liquid water is due to the lower number of water molecules that form the crystal lattice structure of ice, which leaves a large amount of space between water molecules.[32] In contrast, there is no crystal lattice structure in liquid water, which allows more water molecules to occupy the same amount of volume.[32]
Water also has the capacity to absorb energy, giving it a higher specific heat capacity than other solvents such as ethanol.[32] Thus, a large amount of energy is needed to break the hydrogen bonds between water molecules to convert liquid water into water vapor.[32]
As a molecule, water is not completely stable as each water molecule continuously dissociates into hydrogen and hydroxyl ions before reforming into a water molecule again.[32] In pure water, the number of hydrogen ions balances (or equals) the number of hydroxyl ions, resulting in a pH that is neutral.
Organic compounds
Organic compounds are molecules that contain carbon bonded to another element such as hydrogen.[32] With the exception of water, nearly all the molecules that make up each organism contain carbon.[32][33] Carbon can form covalent bonds with up to four other atoms, enabling it to form diverse, large, and complex molecules.[32][33] For example, a single carbon atom can form four single covalent bonds such as in methane, two double covalent bonds such as in carbon dioxide (CO2), or a triple covalent bond such as in carbon monoxide (CO). Moreover, carbon can form very long chains of interconnecting carbon–carbon bonds such as octane or ring-like structures such as glucose.
The simplest form of an organic molecule is the hydrocarbon, which is a large family of organic compounds that are composed of hydrogen atoms bonded to a chain of carbon atoms. A hydrocarbon backbone can be substituted by other elements such as oxygen (O), hydrogen (H), phosphorus (P), and sulfur (S), which can change the chemical behavior of that compound.[32] Groups of atoms that contain these elements (O-, H-, P-, and S-) and are bonded to a central carbon atom or skeleton are called functional groups.[32] There are six prominent functional groups that can be found in organisms: amino group, carboxyl group, carbonyl group, hydroxyl group, phosphate group, and sulfhydryl group.[32]
In 1953, the Miller-Urey experiment showed that organic compounds could be synthesized abiotically within a closed system mimicking the conditions of early Earth, thus suggesting that complex organic molecules could have arisen spontaneously in early Earth (see abiogenesis).[34][32]
Macromolecules
Macromolecules are large molecules made up of smaller molecular subunits that are joined.[35] Small molecules such as sugars, amino acids, and nucleotides can act as single repeating units called monomers to form chain-like molecules called polymers via a chemical process called condensation.[36] For example, amino acids can form polypeptides whereas nucleotides can form strands of nucleic acid. Polymers make up three of the four macromolecules (polysaccharides, lipids, proteins, and nucleic acids) that are found in all organisms. Each of these macromolecules plays a specialized role within any given cell.
Carbohydrates (or sugar) are molecules with the molecular formula (CH2O)n, with n being the number of carbon-hydrate groups.[37] They include monosaccharides (monomer), oligosaccharides (small polymers), and polysaccharides (large polymers). Monosaccharides can be linked together by glycosidic linkages, a type of covalent bond.[37] When two monosaccharides such as glucose and fructose are linked together, they can form a disaccharide such as sucrose.[37] When many monosaccharides are linked together, they can form an oligosaccharide or a polysaccharide, depending on the number of monosaccharides. Polysaccharides can vary in function. Monosaccharides such as glucose can be a source of energy and some polysaccharides can serve as storage material that can be hydrolyzed to provide cells with sugar.
Lipids are the only class of macromolecules that are not made up of polymers. The most biologically important lipids are steroids, phospholipids, and fats.[36] These lipids are organic compounds that are largely nonpolar and hydrophobic.[38] Steroids are organic compounds that consist of four fused rings.[38] Phospholipids consist of glycerol that is linked to a phosphate group and two hydrocarbon chains (or fatty acids).[38] The glycerol and phosphate group together constitute the polar and hydrophilic (or head) region of the molecule whereas the fatty acids make up the nonpolar and hydrophobic (or tail) region.[38] Thus, when in water, phospholipids tend to form a phospholipid bilayer whereby the hydrophobic heads face outwards to interact with water molecules. Conversely, the hydrophobic tails face inwards towards other hydrophobic tails to avoid contact with water.[38]
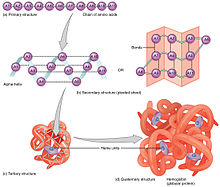
Proteins are the most diverse of the macromolecules, which include enzymes, transport proteins, large signaling molecules, antibodies, and structural proteins. The basic unit (or monomer) of a protein is an amino acid, which has a central carbon atom that is covalently bonded to a hydrogen atom, an amino group, a carboxyl group, and a side chain (or R-group, "R" for residue).[35] There are twenty amino acids that make up the building blocks of proteins, with each amino acid having its own unique side chain.[35] The polarity and charge of the side chains affect the solubility of amino acids. An amino acid with a side chain that is polar and electrically charged is soluble as it is hydrophilic whereas an amino acid with a side chain that lacks a charged or an electronegative atom is hydrophobic and therefore tends to coalesce rather than dissolve in water.[35] Proteins have four distinct levels of organization (primary, secondary, tertiary, and quartenary). The primary structure consists of a unique sequence of amino acids that are covalently linked together by peptide bonds.[35] The side chains of the individual amino acids can then interact with each other, giving rise to the secondary structure of a protein.[35] The two common types of secondary structures are alpha helices and beta sheets.[35] The folding of alpha helices and beta sheets gives a protein its three-dimensional or tertiary structure. Finally, multiple tertiary structures can combine to form the quaternary structure of a protein.
Nucleic acids are polymers made up of monomers called nucleotides.[39] Their function is to store, transmit, and express hereditary information.[36] Nucleotides consist of a phosphate group, a five-carbon sugar, and a nitrogenous base. Ribonucleotides, which contain ribose as the sugar, are the monomers of ribonucleic acid (RNA). In contrast, deoxyribonucleotides contain deoxyribose as the sugar and are constitute the monomers of deoxyribonucleic acid (DNA). RNA and DNA also differ with respect to one of their bases.[39] There are two types of bases: purines and pyrimidines.[39] The purines include guanine (G) and adenine (A) whereas the pyrimidines consist of cytosine (C), uracil (U), and thymine (T). Uracil is used in RNA whereas thymine is used in DNA. Taken together, when the different sugar and bases are take into consideration, there are eight distinct nucleotides that can form two types of nucleic acids: DNA (A, G, C, and T) and RNA (A, G, C, and U).[39]
Cells
Cell theory states that cells are the fundamental units of life, that all living things are composed of one or more cells, and that all cells arise from preexisting cells through cell division.[40] Most cells are very small, with diameters ranging from 1 to 100 micrometers and are therefore only visible under a light or electron microscope.[41] There are generally two types of cells: eukaryotic cells, which contain a nucleus, and prokaryotic cells, which do not. Prokaryotes are single-celled organisms such as bacteria, whereas eukaryotes can be single-celled or multicellular. In multicellular organisms, every cell in the organism's body is derived ultimately from a single cell in a fertilized egg.
Cell structure
Every cell is enclosed within a cell membrane that separates its cytoplasm from the extracellular space.[42] A cell membrane consists of a lipid bilayer, including cholesterols that sit between phospholipids to maintain their fluidity at various temperatures. Cell membranes are semipermeable, allowing small molecules such as oxygen, carbon dioxide, and water to pass through while restricting the movement of larger molecules and charged particles such as ions.[43] Cell membranes also contains membrane proteins, including integral membrane proteins that go across the membrane serving as membrane transporters, and peripheral proteins that loosely attach to the outer side of the cell membrane, acting as enzymes shaping the cell.[44] Cell membranes are involved in various cellular processes such as cell adhesion, storing electrical energy, and cell signalling and serve as the attachment surface for several extracellular structures such as a cell wall, glycocalyx, and cytoskeleton.
Within the cytoplasm of a cell, there are many biomolecules such as proteins and nucleic acids.[45] In addition to biomolecules, eukaryotic cells have specialized structures called organelles that have their own lipid bilayers or are spatially units.[46] These organelles include the cell nucleus, which contains most of the cell's DNA, or mitochondria, which generates adenosine triphosphate (ATP) to power cellular processes. Other organelles such as endoplasmic reticulum and Golgi apparatus play a role in the synthesis and packaging of proteins, respectively. Biomolecules such as proteins can be engulfed by lysosomes, another specialized organelle. Plant cells have additional organelles that distinguish them from animal cells such as a cell wall that provides support for the plant cell, chloroplasts that harvest sunlight energy to produce sugar, and vacuoles that provide storage and structural support as well as being involved in reproduction and breakdown of plant seeds.[46] Eukaryotic cells also have cytoskeleton that is made up of microtubules, intermediate filaments, and microfilaments, all of which provide support for the cell and are involved in the movement of the cell and its organelles.[46] In terms of their structural composition, the microtubules are made up of tubulin (e.g., α-tubulin and β-tubulin whereas intermediate filaments are made up of fibrous proteins.[46] Microfilaments are made up of actin molecules that interact with other strands of proteins.[46]
Metabolism
All cells require energy to sustain cellular processes. Energy is the capacity to do work, which, in thermodynamics, can be calculated using Gibbs free energy. According to the first law of thermodynamics, energy is conserved, i.e., cannot be created or destroyed. Hence, chemical reactions in a cell do not create new energy but are involved instead in the transformation and transfer of energy.[47] Nevertheless, all energy transfers lead to some loss of usable energy, which increases entropy (or state of disorder) as stated by the second law of thermodynamics. As a result, an organism requires continuous input of energy to maintain a low state of entropy. In cells, energy can be transferred as electrons during redox (reduction–oxidation) reactions, stored in covalent bonds, and generated by the movement of ions (e.g., hydrogen, sodium, potassium) across a membrane.
Metabolism is the set of life-sustaining chemical reactions in organisms. The three main purposes of metabolism are: the conversion of food to energy to run cellular processes; the conversion of food/fuel to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolic reactions may be categorized as catabolic—the breaking down of compounds (for example, the breaking down of glucose to pyruvate by cellular respiration); or anabolic—the building up (synthesis) of compounds (such as proteins, carbohydrates, lipids, and nucleic acids). Usually, catabolism releases energy, and anabolism consumes energy.
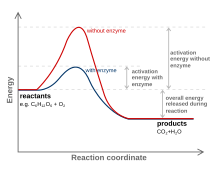
The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, each step being facilitated by a specific enzyme. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts—they allow a reaction to proceed more rapidly without being consumed by it—by reducing the amount of activation energy needed to convert reactants into products. Enzymes also allow the regulation of the rate of a metabolic reaction, for example in response to changes in the cell's environment or to signals from other cells.
Cellular respiration
Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products.[48] The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it clearly does not resemble one when it occurs in a cell because of the slow, controlled release of energy from the series of reactions.
Sugar in the form of glucose is the main nutrient used by animal and plant cells in respiration. Cellular respiration involving oxygen is called aerobic respiration, which has four stages: glycolysis, citric acid cycle (or Krebs cycle), electron transport chain, and oxidative phosphorylation.[49] Glycolysis is a metabolic process that occurs in the cytoplasm whereby glucose is converted into two pyruvates, with two net molecules of ATP being produced at the same time.[49] Each pyruvate is then oxidized into acetyl-CoA by the pyruvate dehydrogenase complex, which also generates NADH and carbon dioxide. Acetyl-Coa enters the citric acid cycle, which takes places inside the mitochondrial matrix. At the end of the cycle, the total yield from 1 glucose (or 2 pyruvates) is 6 NADH, 2 FADH2, and 2 ATP molecules. Finally, the next stage is oxidative phosphorylation, which in eukaryotes, occurs in the mitochondrial cristae. Oxidative phosphorylation comprises the electron transport chain, which is a series of four protein complexes that transfer electrons from one complex to another, thereby releasing energy from NADH and FADH2 that is coupled to the pumping of protons (hydrogen ions) across the inner mitochondrial membrane (chemiosmosis), which generates a proton motive force.[49] Energy from the proton motive force drives the enzyme ATP synthase to synthesize more ATPs by phosphorylating ADPs. The transfer of electrons terminates with molecular oxygen being the final electron acceptor.
If oxygen were not present, pyruvate would not be metabolized by cellular respiration but undergoes a process of fermentation. The pyruvate is not transported into the mitochondrion but remains in the cytoplasm, where it is converted to waste products that may be removed from the cell. This serves the purpose of oxidizing the electron carriers so that they can perform glycolysis again and removing the excess pyruvate. Fermentation oxidizes NADH to NAD+ so it can be re-used in glycolysis. In the absence of oxygen, fermentation prevents the buildup of NADH in the cytoplasm and provides NAD+ for glycolysis. This waste product varies depending on the organism. In skeletal muscles, the waste product is lactic acid. This type of fermentation is called lactic acid fermentation. In strenuous exercise, when energy demands exceed energy supply, the respiratory chain cannot process all of the hydrogen atoms joined by NADH. During anaerobic glycolysis, NAD+ regenerates when pairs of hydrogen combine with pyruvate to form lactate. Lactate formation is catalyzed by lactate dehydrogenase in a reversible reaction. Lactate can also be used as an indirect precursor for liver glycogen. During recovery, when oxygen becomes available, NAD+ attaches to hydrogen from lactate to form ATP. In yeast, the waste products are ethanol and carbon dioxide. This type of fermentation is known as alcoholic or ethanol fermentation. The ATP generated in this process is made by substrate-level phosphorylation, which does not require oxygen.
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organism's metabolic activities via cellular respiration. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water.[50][51][52] In most cases, oxygen is also released as a waste product. Most plants, algae, and cyanobacteria perform photosynthesis, which is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth.[53]
Photosynthesis has four stages: Light absorption, electron transport, ATP synthesis, and carbon fixation.[49] Light absorption is the initial step of photosynthesis whereby light energy is absorbed by chlorophyll pigments attached to proteins in the thylakoid membranes. The absorbed light energy is used to remove electrons from a donor (water) to a primary electron acceptor, a quinone designated as Q. In the second stage, electrons move from the quinone primary electron acceptor through a series of electron carriers until they reach a final electron acceptor, which is usually the oxidized form of NADP+, which is reduced to NADPH, a process that takes place in a protein complex called photosystem I (PSI). The transport of electrons is coupled to the movement of protons (or hydrogen) from the stroma to the thylakoid membrane, which forms a pH gradient across the membrane as hydrogen becomes more concentrated in the lumen than in the stroma. This is analogous to the proton-motive force generated across the inner mitochondrial membrane in aerobic respiration.[49]
During the third stage of photosynthesis, the movement of protons down their concentration gradients from the thylakoid lumen to the stroma through the ATP synthase is coupled to the synthesis of ATP by that same ATP synthase.[49] The NADPH and ATPs generated by the light-dependent reactions in the second and third stages, respectively, provide the energy and electrons to drive the synthesis of glucose by fixing atmospheric carbon dioxide into existing organic carbon compounds, such as ribulose bisphosphate (RuBP) in a sequence of light-independent (or dark) reactions called the Calvin cycle.[54]
Cell signaling
Cell signaling (or communication) is the ability of cells to receive, process, and transmit signals with its environment and with itself.[55][56] Signals can be non-chemical such as light, electrical impulses, and heat, or chemical signals (or ligands) that interact with receptors, which can be found embedded in the cell membrane of another cell or located deep inside a cell.[57][56] There are generally four types of chemical signals: autocrine, paracrine, juxtacrine, and hormones.[57] In autocrine signaling, the ligand affects the same cell that releases it. Tumor cells, for example, can reproduce uncontrollably because they release signals that initiate their own self-division. In paracrine signaling, the ligand diffuses to nearby cells and affect them. For example, brain cells called neurons release ligands called neurotransmitters that diffuse across a synaptic cleft to bind with a receptor on an adjacent cell such as another neuron or muscle cell. In juxtacrine signaling, there is direct contact between the signaling and responding cells. Finally, hormones are ligands that travel through the circulatory systems of animals or vascular systems of plants to reach their target cells. Once a ligand binds with a receptor, it can influence the behavior of another cell, depending on the type of receptor. For instance, neurotransmitters that bind with an inotropic receptor can alter the excitability of a target cell. Other types of receptors include protein kinase receptors (e.g., receptor for the hormone insulin) and G protein-coupled receptors. Activation of G protein-coupled receptors can initiate second messenger cascades. The process by which a chemical or physical signal is transmitted through a cell as a series of molecular events is called signal transduction
Cell cycle
The cell cycle is a series of events that take place in a cell that cause it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and the subsequent partitioning of its cytoplasm into two daughter cells in a process called cell division.[58] In eukaryotes (i.e., animal, plant, fungal, and protist cells), there are two distinct types of cell division: mitosis and meiosis.[59] Mitosis is part of the cell cycle, in which replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. In general, mitosis (division of the nucleus) is preceded by the S stage of interphase (during which the DNA is replicated) and is often followed by telophase and cytokinesis; which divides the cytoplasm, organelles and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis all together define the mitotic phase of an animal cell cycle—the division of the mother cell into two genetically identical daughter cells.[60] The cell cycle is a vital process by which a single-celled fertilized egg develops into a mature organism, as well as the process by which hair, skin, blood cells, and some internal organs are renewed. After cell division, each of the daughter cells begin the interphase of a new cycle. In contrast to mitosis, meiosis results in four haploid daughter cells by undergoing one round of DNA replication followed by two divisions.[61] Homologous chromosomes are separated in the first division (meiosis I), and sister chromatids are separated in the second division (meiosis II). Both of these cell division cycles are used in the process of sexual reproduction at some point in their life cycle. Both are believed to be present in the last eukaryotic common ancestor.
Prokaryotes (i.e., archaea and bacteria) can also undergo cell division (or binary fission). Unlike the processes of mitosis and meiosis in eukaryotes, binary fission takes in prokaryotes takes place without the formation of a spindle apparatus on the cell. Before binary fission, DNA in the bacterium is tightly coiled. After it has uncoiled and duplicated, it is pulled to the separate poles of the bacterium as it increases the size to prepare for splitting. Growth of a new cell wall begins to separate the bacterium (triggered by FtsZ polymerization and "Z-ring" formation)[62] The new cell wall (septum) fully develops, resulting in the complete split of the bacterium. The new daughter cells have tightly coiled DNA rods, ribosomes, and plasmids.
Genetics
Inheritance
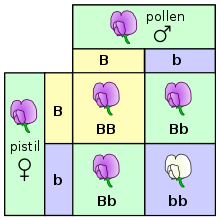
Genetics is the scientific study of inheritance.[63][64][65] Mendelian inheritance, specifically, is the process by which genes and traits are passed on from parents to offspring.[28] It was formulated by Gregor Mendel, based on his work with pea plants in the mid-nineteenth century. Mendel established several principles of inheritance. The first is that genetic characteristics, which are now called alleles, are discrete and have alternate forms (e.g., purple vs. white or tall vs. dwarf), each inherited from one of two parents. Based on his law of dominance and uniformity, which states that some alleles are dominant while others are recessive; an organism with at least one dominant allele will display the phenotype of that dominant allele.[66] Exceptions to this rule include penetrance and expressivity.[28] Mendel noted that during gamete formation, the alleles for each gene segregate from each other so that each gamete carries only one allele for each gene, which is stated by his law of segregation. Heterozygotic individuals produce gametes with an equal frequency of two alleles. Finally, Mendel formulated the law of independent assortment, which states that genes of different traits can segregate independently during the formation of gametes, i.e., genes are unlinked. An exception to this rule would include traits that are sex-linked. Test crosses can be performed to experimentally determine the underlying genotype of an organism with a dominant phenotype.[67] A Punnett square can be used to predict the results of a test cross. The chromosome theory of inheritance, which states that genes are found on chromosomes, was supported by Thomas Morgans's experiments with fruit flies, which established the sex linkage between eye color and sex in these insects.[68] In humans and other mammals (e.g., dogs), it is not feasible or practical to conduct test cross experiments. Instead, pedigrees, which are genetic representations of family trees,[69] are used instead to trace the inheritance of a specific trait or disease through multiple generations.[70]
DNA
A gene is a unit of heredity that corresponds to a region of deoxyribonucleic acid (DNA) that carries genetic information that influences the form or function of an organism in specific ways. DNA is a molecule composed of two polynucleotide chains that coil around each other to form a double helix, which was first described by James Watson and Francis Crick in 1953.[71] It is found as linear chromosomes in eukaryotes, and circular chromosomes in prokaryotes. A chromosome is an organized structure consisting of DNA and histones. The set of chromosomes in a cell and any other hereditary information found in the mitochondria, chloroplasts, or other locations is collectively known as a cell's genome. In eukaryotes, genomic DNA is localized in the cell nucleus, or with small amounts in mitochondria and chloroplasts.[72] In prokaryotes, the DNA is held within an irregularly shaped body in the cytoplasm called the nucleoid.[73] The genetic information in a genome is held within genes, and the complete assemblage of this information in an organism is called its genotype.[74] Genes encode the information needed by cells for the synthesis of proteins, which in turn play a central role in influencing the final phenotype of the organism.
The two polynucleotide strands that make up DNA run in opposite directions to each other and are thus antiparallel. Each strand is composed of nucleotides,[75][76] with each nucleotide containing one of four nitrogenous bases (cytosine [C], guanine [G], adenine [A] or thymine [T]), a sugar called deoxyribose, and a phosphate group. The nucleotides are joined to one another in a chain by covalent bonds between the sugar of one nucleotide and the phosphate of the next, resulting in an alternating sugar-phosphate backbone. It is the sequence of these four bases along the backbone that encodes genetic information. Bases of the two polynucleotide strands are bound together by hydrogen bonds, according to base pairing rules (A with T and C with G), to make double-stranded DNA. The bases are divided into two groups: pyrimidines and purines. In DNA, the pyrimidines are thymine and cytosine whereas the purines are adenine and guanine.
There are grooves that run along the entire length of the double helix due to the uneven spacing of the DNA strands relative to each other.[71] Both grooves differ in size, with the major groove being larger and therefore more accessible to the binding of proteins than the minor groove.[71] The outer edges of the bases are exposed to these grooves and are therefore accessible for additional hydrogen bonding.[71] Because each groove can have two possible base-pair configurations (G-C and A-T), there are four possible base-pair configurations within the entire double helix, each of which is chemically distinct from another.[71] As a result, protein molecules are able to recognize and bind to specific base-pair sequences, which is the basis of specific DNA-protein interactions.
DNA replication is a semiconservative process whereby each strand serves as a template for a new strand of DNA.[71] The process begins with the unwounding of the double helix at an origin of replication, which separates the two strands, thereby making them available as two templates. This is then followed by the binding of the enzyme primase to the template to synthesize a starter RNA (or DNA in some viruses) strand called a primer from the 5' to 3' location.[71] Once the primer is completed, the primase is released from the template, followed by the binding of the enzyme DNA polymerase to the same template to synthesize new DNA. The rate of DNA replication in a living cell was measured as 749 nucleotides added per second under ideal conditions.[77]
DNA replication is not perfect as the DNA polymerase sometimes insert bases that are not complementary to the template (e.g., putting in A in the strand opposite to G in the template strand).[71] In eukaryotes, the initial error or mutation rate is about 1 in 100,000.[71] Proofreading and mismatch repair are the two mechanisms that repair these errors, which reduces the mutation rate to 10−10, particularly before and after a cell cycle.[71]
Mutations are heritable changes in DNA.[71] They can arise spontaneously as a result of replication errors that were not corrected by proofreading or can be induced by an environmental mutagen such as a chemical (e.g., nitrous acid, benzopyrene) or radiation (e.g., x-ray, gamma ray, ultraviolet radiation, particles emitted by unstable isotopes).[71] Mutations can appear as a change in single base or at a larger scale involving chromosomal mutations such as deletions, inversions, or translocations.[71]
In multicellular organisms, mutations can occur in somatic or germline cells.[71] In somatic cells, the mutations are passed on to daughter cells during mitosis.[71] In a germline cell such as a sperm or an egg, the mutation will appear in an organism at fertilization.[71] Mutations can lead to several types of phenotypic effects such as silent, loss-of-function, gain-of-function, and conditional mutations.[71]
Some mutations can be beneficial, as they are a source of genetic variation for evolution.[71] Others can be harmful if they were to result in a loss of function of genes needed for survival.[71] Mutagens such as carcinogens are typically avoided as a matter of public health policy goals.[71] One example is the banning of chlorofluorocarbons (CFC) by the Montreal Protocol, as CFCs tend to deplete the ozone layer, resulting in more ultraviolet radiation from the sun passing through the Earth's upper atmosphere, thereby causing somatic mutations that can lead to skin cancer.[71] Similarly, smoking bans have been enforced throughout the world in an effort to reduce the incidence of lung cancer.[71]
Gene expression
Gene expression is the molecular process by which a genotype gives rise to a phenotype, i.e., observable trait. The genetic information stored in DNA represents the genotype, whereas the phenotype results from the synthesis of proteins that control an organism's structure and development, or that act as enzymes catalyzing specific metabolic pathways. This process is summarized by the central dogma of molecular biology, which was formulated by Francis Crick in 1958.[78][79][80] According to the Central Dogma, genetic information flows from DNA to RNA to protein. Hence, there are two gene expression processes: transcription (DNA to RNA) and translation (RNA to protein).[81] These processes are used by all life—eukaryotes (including multicellular organisms), prokaryotes (bacteria and archaea), and are exploited by viruses—to generate the macromolecular machinery for life.
During transcription, messenger RNA (mRNA) strands are created using DNA strands as a template, which is initiated when RNA polymerase binds to a DNA sequence called a promoter, which instructs the RNA to begin transcription of one of the two DNA strands.[82] The DNA bases are exchanged for their corresponding bases except in the case of thymine (T), for which RNA substitutes uracil (U).[83] In eukaryotes, a large part of DNA (e.g., >98% in humans) contain non-coding called introns, which do not serve as patterns for protein sequences. The coding regions or exons are interspersed along with the introns in the primary transcript (or pre-mRNA).[82] Before translation, the pre-mRNA undergoes further processing whereby the introns are removed (or spliced out), leaving only the spliced exons in the mature mRNA strand.[82]
The translation of mRNA to protein occurs in ribosomes, whereby the transcribed mRNA strand specifies the sequence of amino acids within proteins using the genetic code. Gene products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA.[84][85]














Comments
Post a Comment